Hatch-Waxman Act: How It Made Generic Drugs Accessible and Changed Healthcare
When you pick up a generic pill at the pharmacy and pay a fraction of what the brand-name version costs, you’re seeing the direct result of the Hatch-Waxman Act, a 1984 U.S. law that created a legal pathway for generic drugs to enter the market without repeating expensive clinical trials. Also known as the Drug Price Competition and Patent Term Restoration Act, it was designed to fix a broken system where brand-name companies held monopolies long after their patents expired, and no one could legally make cheaper copies. Before this law, even if a drug’s patent ran out, manufacturers couldn’t easily sell generics because they had to prove safety and effectiveness from scratch — a process that took years and cost millions.
The FDA, the U.S. agency responsible for approving drugs and ensuring their safety became the gatekeeper for this new system. Under the Hatch-Waxman Act, generic makers could file an Abbreviated New Drug Application (ANDA), proving their product is bioequivalent to the brand-name drug — meaning it works the same way in the body — without redoing animal or human trials. This cut approval time from years to months. At the same time, the law gave brand-name companies up to five extra years of market exclusivity to make up for time lost during FDA review, helping them recoup R&D costs. This balance between innovation and access is why the generic drugs, lower-cost versions of brand-name medications that contain the same active ingredients now make up over 90% of prescriptions in the U.S.
The impact? Billions saved every year by patients, insurers, and Medicare. A drug that once cost $300 a month might now be $10 as a generic — and it’s just as effective. You’ll find posts here that explain how this law made medications like metformin, amoxicillin, and birth control pills affordable for millions. You’ll also see how it connects to today’s debates: why some specialty drugs still cost too much, how biosimilars are following the same path, and why counterfeit drugs sometimes slip through the cracks because the system is overloaded. This isn’t just history — it’s the reason your prescription is cheaper today. Below, you’ll find real-world examples of how Hatch-Waxman shaped drug access, from generic specialty drugs to cross-border pharmacy rules and why some medications are still out of reach despite the law.

Generic Drug Patents: How Exclusivity Periods Vary Across Countries
Generic drug exclusivity periods vary widely across countries, with the U.S., EU, and others using complex rules to delay cheaper versions. Learn how patents, data exclusivity, and legal strategies shape drug access and pricing worldwide.
Detail
FDA Authorization of Generics: Legal Basis and Approval Process
The FDA approves generic drugs through the Hatch-Waxman Act's ANDA pathway, ensuring they match brand-name drugs in safety, strength, and effectiveness. Over 90% of U.S. prescriptions are for generics, saving billions annually.
Detail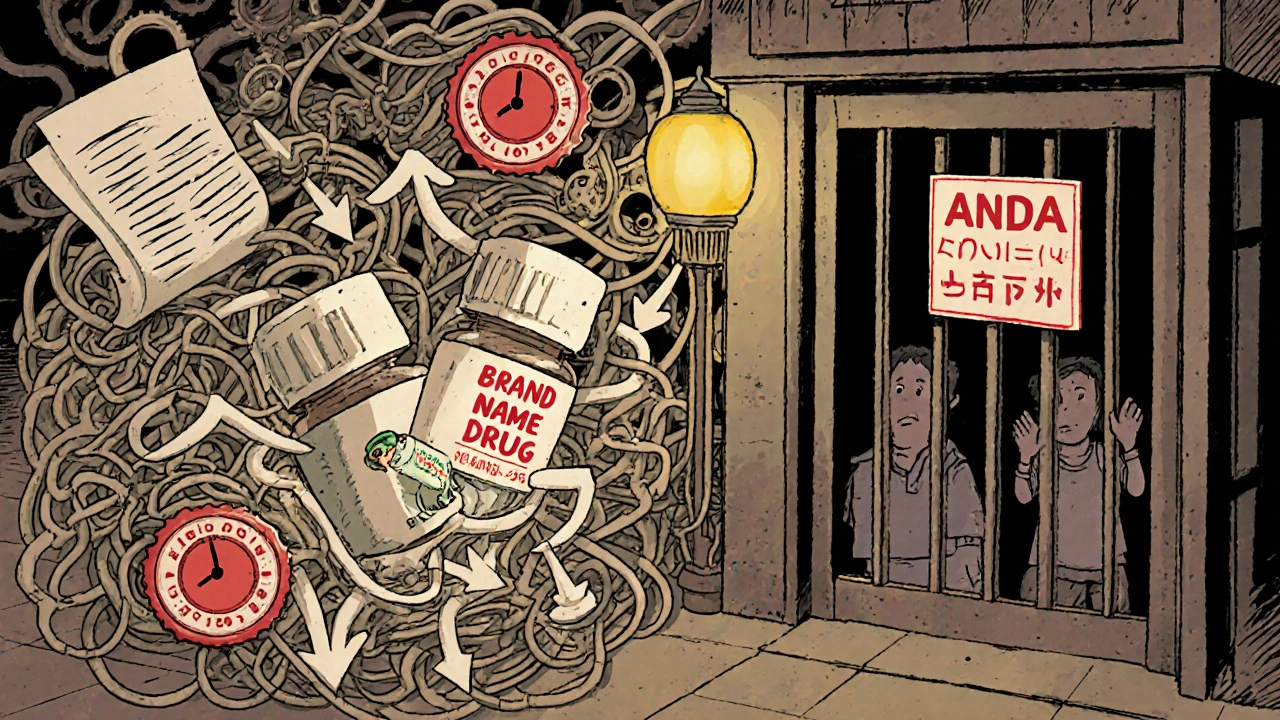
Generic Drug Availability: How Long After Patent Expiration Do They Actually Hit Shelves?
Generic drugs often take years to appear after a brand-name drug’s patent expires due to legal delays, patent thickets, and regulatory hurdles. Learn why cheaper versions don’t hit shelves right away - and what’s being done about it.
Detail