ANDA Approval: What It Means for Generic Drugs and Your Wallet
When you pick up a generic pill at the pharmacy, chances are it got here because of ANDA approval, a streamlined process by the U.S. Food and Drug Administration that allows generic versions of brand-name drugs to be sold legally. Also known as Abbreviated New Drug Application, it’s the backbone of affordable medicine in the U.S. This isn’t just paperwork—it’s the reason you’re paying $4 for metformin instead of $300 for Glucophage. The FDA doesn’t demand new clinical trials for generics. Instead, they require proof that the generic matches the brand in active ingredient, strength, dosage form, and how it works in your body. That’s it. No need to re-prove safety or effectiveness. The brand already did that. The generic just has to copy it accurately.
That’s where generic drugs, medications that contain the same active ingredient as a brand-name drug but are sold under their chemical name. Also known as non-brand drugs, they come into play. These aren’t knockoffs. They’re legally required to be bioequivalent—meaning they deliver the same amount of medicine into your bloodstream at the same rate. That’s why a generic version of Lamivudine or Metoprolol works just as well as the brand. And why companies like those behind Trimox or Combipres can offer cheaper alternatives without cutting corners. The FDA’s pharmaceutical regulation, the system of rules and inspections that ensure drugs are safe, effective, and consistently manufactured makes sure of that. They audit manufacturing sites, check for contamination, and monitor for fake or substandard products. That’s why seized counterfeit medications are such a big deal—they bypass this whole system.
And it’s not just about price. drug affordability, how accessible and financially manageable medications are for patients and insurers depends on ANDA approval. Without it, generics wouldn’t exist in the numbers they do. That means fewer people skip doses because they can’t pay. Fewer people choose between meds and rent. It’s why cross-border pharmacy services in the EU work the way they do—people are chasing the same thing: lower cost, same effect. Even specialty pharmacies rely on this system to dispense generic versions of complex drugs. The entire structure of modern pharmacy rests on this one process: prove you’re the same, get approved fast, sell cheap.
What you’ll find below are real stories and breakdowns of how this system touches everyday life—from the safety of topical creams during pregnancy to the rise of counterfeit Ozempic pills. You’ll see how ANDA approval enables alternatives to Geriforte Syrup, makes Glucophage cheaper, and lets patients switch from Kaletra to a generic HIV combo without losing effectiveness. It’s the quiet engine behind every affordable prescription. And if you’ve ever wondered why your pill looks different but works the same—now you know why.

FDA Authorization of Generics: Legal Basis and Approval Process
The FDA approves generic drugs through the Hatch-Waxman Act's ANDA pathway, ensuring they match brand-name drugs in safety, strength, and effectiveness. Over 90% of U.S. prescriptions are for generics, saving billions annually.
Detail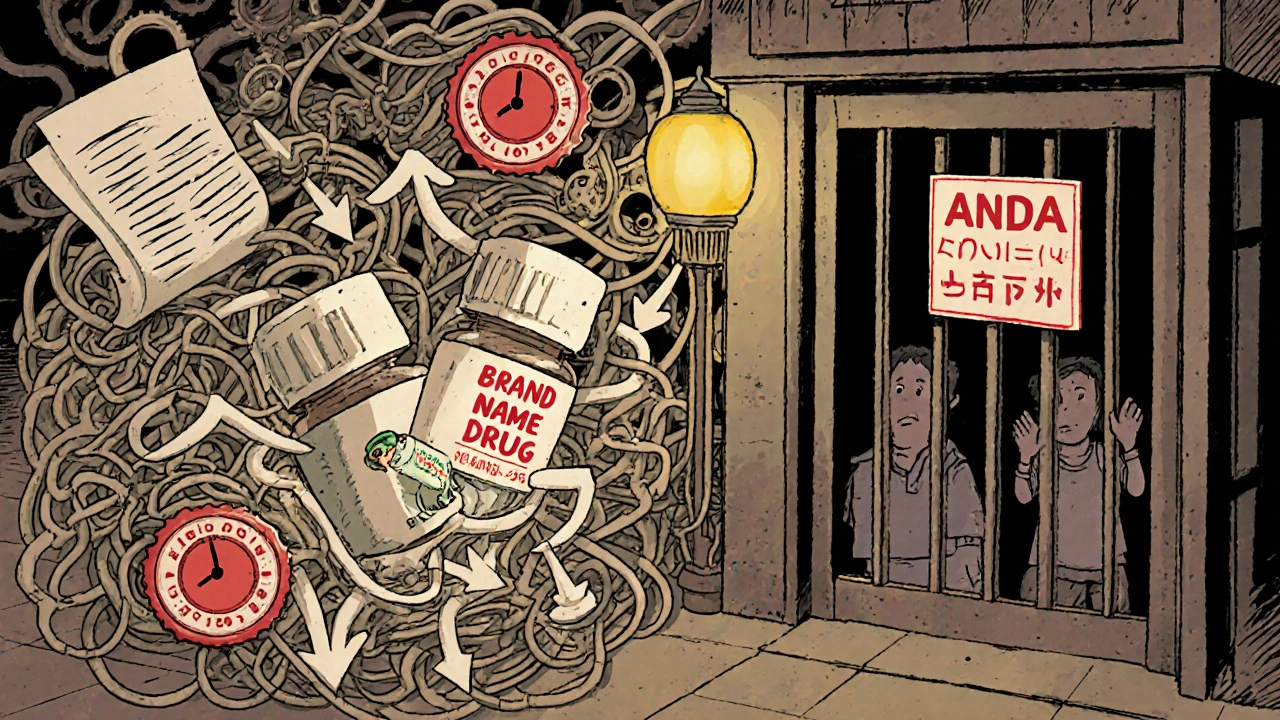
Generic Drug Availability: How Long After Patent Expiration Do They Actually Hit Shelves?
Generic drugs often take years to appear after a brand-name drug’s patent expires due to legal delays, patent thickets, and regulatory hurdles. Learn why cheaper versions don’t hit shelves right away - and what’s being done about it.
Detail