Most people assume that if a drug is approved by the FDA or similar agencies, it’s completely safe. But the truth is, some of the most dangerous drug interactions aren’t found until after millions of people start taking the medicine. These hidden risks - like a common antifungal turning a cholesterol drug into a muscle-destroying threat - don’t show up in clinical trials. They emerge only when real people, with real health conditions and real lifestyles, use the drug long-term, alongside other medications, foods, or supplements.
Why Clinical Trials Miss These Risks
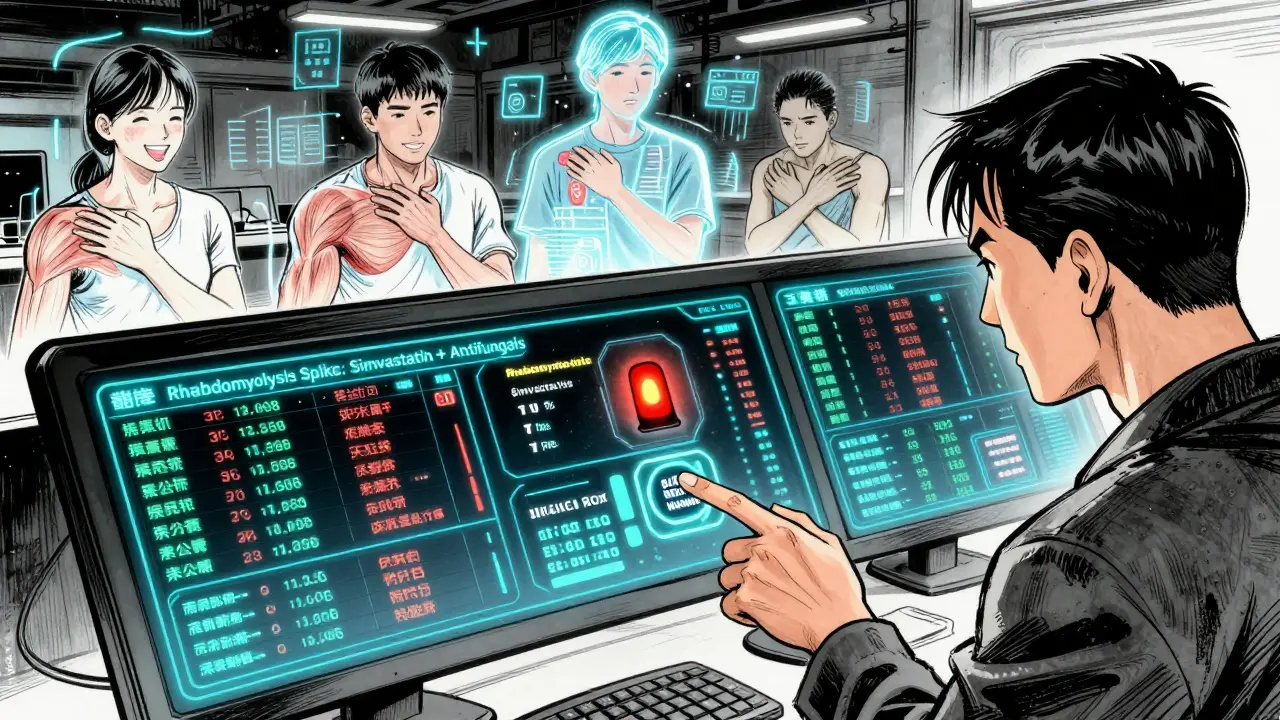
Clinical trials are designed to prove a drug works, not to catch every possible side effect. They typically involve 1,000 to 5,000 people over 6 to 12 months. That’s not enough to spot rare reactions or interactions that only happen after years of use. Trials also exclude many real-world patients: older adults with multiple conditions, pregnant women, children, and people taking five or more medications. These are exactly the people most at risk.Take simvastatin (Zocor), a widely prescribed statin. In trials, it looked safe. But after millions of prescriptions, doctors noticed patients on this drug were developing severe muscle damage - rhabdomyolysis - when they also took antifungals like fluconazole. Why? Fluconazole blocks the liver enzyme CYP3A4, which is responsible for breaking down simvastatin. Without that breakdown, simvastatin builds up to 3 to 10 times its normal level in the blood. That’s enough to destroy muscle tissue and damage kidneys. This interaction wasn’t flagged until after the drug was on the market for over a decade.
Same with grapefruit juice and atorvastatin (Lipitor). A single glass of grapefruit juice can raise Lipitor levels by up to 15 times. That’s not a myth - it’s science. Yet, many patients aren’t warned. A Reddit user in 2022 shared how they ended up in the ER with kidney failure because their doctor never mentioned grapefruit. They thought it was just a healthy drink.
Three Types of Hidden Drug Interactions
Post-market discoveries fall into three main categories:- Drug-drug interactions: One medication changes how another works. This is the most common. Examples include blood thinners like apixaban (Eliquis) interacting with St. John’s Wort, which can cause life-threatening bleeding. Or ciprofloxacin (Cipro) raising the risk of dangerous heart rhythm changes when taken with certain blood pressure drugs.
- Drug-condition interactions: A health condition makes a drug more dangerous. For example, patients with kidney disease can’t clear certain antibiotics like vancomycin properly, leading to toxicity. Or someone with liver disease taking acetaminophen (Tylenol) - even at normal doses - can suffer liver failure.
- Drug-food interactions: What you eat or drink affects your medication. Besides grapefruit, dairy products can block absorption of antibiotics like tetracycline. Alcohol can intensify drowsiness from benzodiazepines or trigger dangerous drops in blood sugar with diabetes meds.
These aren’t theoretical risks. The FDA’s FAERS database shows over 2,800 reports of rhabdomyolysis tied to statin-azole interactions between 2015 and 2020. Nearly 40% of those involved simvastatin. And that’s just what got reported - experts estimate 90% to 95% of adverse events go unreported.
How These Risks Are Found After Approval
Once a drug is on the market, monitoring kicks into high gear. The FDA’s Sentinel Initiative tracks over 300 million patient records across hospitals, insurers, and pharmacies. It looks for unusual spikes in hospitalizations or lab results that might point to a hidden danger. If a pattern emerges - say, a sudden rise in liver damage cases among users of a new diabetes drug - regulators investigate.Pharmacists and doctors also play a key role. The Naranjo Algorithm is a tool used by specialists to determine if a reaction was truly caused by a drug interaction. It looks at timing: Did symptoms start after taking the new drug? Did they improve when it was stopped? Could something else explain it? It’s not perfect, but it helps separate real risks from coincidence.
Technology is accelerating detection. Oracle Health Sciences’ AI platform, approved by the FDA in early 2023, can analyze 10,000 adverse event reports daily with 92.7% accuracy. The European Medicines Agency’s EudraVigilance system now uses machine learning to cut signal detection time from 18 months to just 45 days. These tools are catching things humans might miss.

Real Consequences: Drugs Pulled and Warnings Added
Post-market discoveries have led to major changes:- Terfenadine (Seldane), an allergy drug, was pulled in 1998 after it caused fatal heart rhythms when taken with ketoconazole or grapefruit juice.
- Pergolide (Permax), used for Parkinson’s, was withdrawn in 2007 after links to heart valve damage surfaced in over a million patient-years of use.
- Benfluorex (Mediator), a weight-loss drug in France, was pulled in 2009 after 5 million people used it for 30 years - and thousands developed heart valve damage.
- Exalgo, an extended-release painkiller, got a black box warning in 2012 after reports of fatal overdoses when taken with alcohol - a risk not seen in trials.
According to a 2021 FDA analysis, nearly one-third of all new drugs approved over a 10-year period received a major safety update after launch - either a black box warning, a market withdrawal, or a public safety alert. Almost 20% of new drugs got a black box warning only after they were widely used. And 4% were pulled entirely.
Why This Matters to You
If you take more than one medication - and most adults over 50 do - you’re at risk. A 2022 IQVIA study found that 15% to 20% of hospital admissions in the U.S. are due to preventable adverse drug events, and a large chunk of those involve interactions. The cost? Over $1 billion a year in the U.S. alone.But here’s the good news: you don’t have to wait for a warning to come out. You can protect yourself.
- Always tell your doctor and pharmacist everything you take - including vitamins, herbs, and OTC meds. St. John’s Wort might seem harmless, but it interferes with over 50 drugs.
- Use free tools like GoodRx’s interaction checker. Thousands of users say it’s saved them. One person reported it caught a dangerous interaction between ciprofloxacin and their blood pressure pill - their pharmacist confirmed it could have caused QT prolongation, a potentially fatal heart rhythm issue.
- Ask: “Could this interact with anything else I’m taking?” Don’t assume your doctor knows all your meds. Many patients don’t mention supplements.
- Check labels. If a drug has a black box warning, read it. It’s the strongest warning the FDA gives.
The Future of Drug Safety
The system is getting smarter. The FDA now requires post-approval studies for nearly half of all new drugs. By 2025, 68% of big pharmaceutical companies plan to use blockchain to improve reporting accuracy. The NIH’s Pharmacogenomics Research Network is studying how your genes affect how you respond to drugs - meaning someday, your prescription might be tailored to your DNA.But technology alone won’t fix this. Labeling is still messy. Many drug inserts bury interaction warnings in fine print. Pharmacists are overwhelmed. Patients are left guessing.
Real change means better communication - not just between doctors and patients, but between systems. The FDA’s Drug Interaction API, used by 2.5 million daily queries from electronic health records, is a step forward. But until warnings are clear, consistent, and front-and-center, people will keep getting hurt.
What You Can Do Today
Don’t wait for a drug to be pulled or a warning to appear. Take action now:- Make a complete list of every medication, supplement, and herbal product you take - including doses and how often.
- Bring that list to every doctor and pharmacist visit.
- Use a free online checker like GoodRx or Medscape before starting any new drug.
- If you experience unexplained muscle pain, weakness, fatigue, or dark urine after starting a new med - get it checked immediately. These could be signs of rhabdomyolysis.
- Ask your pharmacist: “Is there anything here I should avoid eating, drinking, or taking with this?” They’re trained to catch what doctors miss.
The life of a drug doesn’t end when it’s approved. It begins. And your safety depends on staying informed - not just trusting the label.
What does it mean if a drug gets a black box warning after approval?
A black box warning is the strongest safety alert the FDA can issue. It means serious or life-threatening risks were found after the drug was already on the market - often due to drug interactions, long-term use, or effects in vulnerable populations. It doesn’t mean the drug is unsafe for everyone, but it requires extra caution. Doctors must discuss these risks with patients before prescribing.
Can over-the-counter drugs and supplements cause dangerous interactions?
Yes. Many people think OTC meds and supplements are harmless, but that’s not true. St. John’s Wort can reduce the effectiveness of birth control, antidepressants, and blood thinners. Calcium supplements can block absorption of thyroid meds. Even common painkillers like ibuprofen can raise blood pressure and increase bleeding risk when taken with blood thinners. Always check interactions, even with non-prescription items.
Why aren’t all drug interactions listed on the label?
Not all interactions are known at the time of approval, and some are too rare to be detected in trials. Also, labeling space is limited, and manufacturers sometimes prioritize listing the most common side effects. Plus, interactions can emerge years later. That’s why post-market surveillance exists - to catch what initial testing missed. Always consult your pharmacist for the most up-to-date interaction info.
How long does it take to find a dangerous interaction after a drug is approved?
It varies. Some are found within months, like the Exalgo-alcohol interaction detected after 18 months. Others take years - like benfluorex, which caused heart damage in thousands over 30 years. The average time for a major safety event to be detected post-market is 2 to 5 years, but it can be much longer. That’s why ongoing monitoring is critical.
Are there tools I can use to check for drug interactions myself?
Yes. Free tools like GoodRx, Medscape’s Drug Interaction Checker, and the FDA’s FAERS database (accessible to the public) can help. Many pharmacy apps also include interaction alerts. These aren’t perfect, but they’re far better than guessing. Always double-check with your pharmacist - they have access to more detailed databases and clinical experience.






Henry Marcus
December 19, 2025 AT 13:59Matt Davies
December 21, 2025 AT 11:06Meenakshi Jaiswal
December 22, 2025 AT 00:44mark shortus
December 23, 2025 AT 03:28Takeysha Turnquest
December 23, 2025 AT 03:50Aboobakar Muhammedali
December 24, 2025 AT 20:14Laura Hamill
December 25, 2025 AT 00:26Alana Koerts
December 25, 2025 AT 02:31Dikshita Mehta
December 25, 2025 AT 04:47pascal pantel
December 25, 2025 AT 19:12Gloria Parraz
December 26, 2025 AT 01:17Sahil jassy
December 26, 2025 AT 21:53Kathryn Featherstone
December 28, 2025 AT 09:30Nicole Rutherford
December 28, 2025 AT 22:18Chris Clark
December 30, 2025 AT 18:36