It’s a common fear: you switch from your brand-name pill to a cheaper generic, and suddenly you feel off. Headaches. Nausea. Sleeplessness. You start wondering - did the generic cause a bad interaction? Did it react differently with your other meds? The truth is, generic drugs don’t interact differently than their brand-name cousins - not because of the active ingredient. But here’s the catch: people still report problems. And those problems are real, even when the science says they shouldn’t be.
Why You Think Generics Cause Unexpected Reactions
Most people assume generics are just knockoffs. They’re not. By law, a generic drug must contain the exact same active ingredient, in the same strength, and work the same way in your body as the brand-name version. The U.S. Food and Drug Administration (FDA) requires this. In fact, between 2018 and 2023, 97.4% of approved generics matched the brand-name drug’s bioavailability within a tight 80%-125% range - a margin too small to matter for most medications. So why do some patients swear their generic version gives them side effects the brand never did? Three big reasons:- Inactive ingredients - things like fillers, dyes, or preservatives - can differ between brands. One generic version of levothyroxine used lactose as a filler; a patient allergic to dairy had stomach cramps. The brand version used a different filler. The active drug? Identical. The reaction? Real.
- Nocebo effect - if you believe generics are inferior, your brain can make you feel worse. A Harvard study found patients reported more side effects simply because they knew they were taking a cheaper version. It’s the opposite of the placebo effect.
- Switching between generic manufacturers - your pharmacy might switch from one generic maker to another without telling you. Each may use different fillers. One batch might have sodium benzoate; the next might use a different salt form. For sensitive people, that’s enough to trigger discomfort.
What Actually Causes Dangerous Drug Interactions
Drug interactions happen because of the active ingredient - not whether it’s branded or generic. Here are the real culprits:- Drug-drug interactions - like fluconazole (an antifungal) making simvastatin (a cholesterol drug) 300-400% more potent. That’s dangerous. It doesn’t matter if you’re on Lipitor or its generic. The interaction is the same.
- Drug-food interactions - grapefruit juice blocks enzymes that break down certain drugs. One grapefruit can raise blood levels of calcium channel blockers by 70%. Again, brand or generic? Irrelevant. The active ingredient reacts the same way.
- Drug-condition interactions - diphenhydramine (Benadryl) can raise eye pressure, which is risky if you have glaucoma. This isn’t about the pill’s price tag. It’s about the chemical in your bloodstream.
The FDA requires generic drug labels to list the exact same interaction warnings as the brand-name version. And they do. In 2022, compliance was 100%.
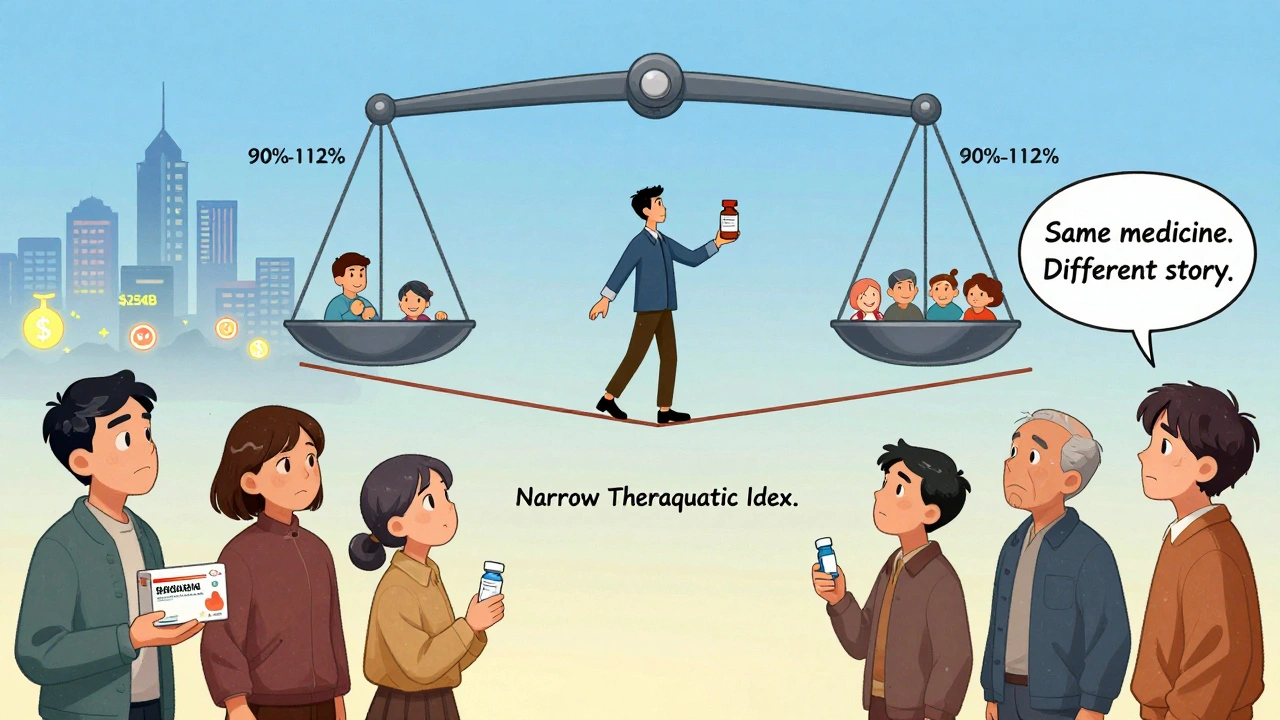
The Real Risk: Narrow Therapeutic Index Drugs
Some drugs are like walking a tightrope. A tiny change in blood level - even 10% - can mean the difference between working perfectly and causing harm. These are called narrow therapeutic index (NTI) drugs. Examples: warfarin (blood thinner), levothyroxine (thyroid), digoxin (heart), and phenytoin (seizure control). For these, the FDA demands tighter bioequivalence: 90%-112% instead of 80%-125%. That’s stricter. But even then, problems pop up - not because the generic is weaker, but because patients switch manufacturers too often. A 2023 study tracked 112 reports of INR fluctuations (a blood test for warfarin) on Inspire.com. Pharmacists followed up. 89% of the cases were linked to dietary changes - eating more greens, skipping meals, drinking alcohol - not the generic pill. Yet patients blamed the switch. Hospitals know this. 76% of U.S. hospitals use just one generic manufacturer for NTI drugs. Why? Consistency. If you’re on warfarin, staying with the same generic batch reduces variables. Your doctor might not tell you this - but it’s standard practice.
Why the Perception Persists - And Why It Costs Money
A 2022 Kaiser Family Foundation survey found 47% of patients believe generics work differently than brand-name drugs. Nearly 3 in 10 say they get more side effects from generics. But when researchers checked medical records, only 3.7% of those reports matched actual physiological differences. The rest? Psychology, confusion, or misattribution. Someone switches from brand to generic, then gets sick from the flu. They assume the generic caused it. Or they read a Reddit thread where someone else had a bad reaction. Suddenly, the generic is the villain. This perception has real costs. In 2023, $8.7 billion was spent in the U.S. on unnecessary brand-name prescriptions - because people feared generics. That’s 1.2 million extra prescriptions filled at full price, just because of fear.What You Can Do: Practical Safety Steps
You don’t need to avoid generics. But you do need to be smart:- Ask your pharmacist - if you’re on warfarin, levothyroxine, or another NTI drug, ask if your pharmacy uses the same generic manufacturer each time. Request consistency.
- Check the pill’s appearance - if your pill suddenly looks different (color, shape, markings), ask why. It might be a different generic. That’s fine - but know it’s happened.
- Track your symptoms - write down when you feel off. Did it start after a refill? After eating grapefruit? After changing your diet? Correlation isn’t causation, but it helps your doctor figure it out.
- Don’t assume it’s the generic - if you feel worse after switching, don’t jump to blame the pill. Talk to your doctor. Maybe your thyroid dose needs adjusting. Maybe your liver enzymes changed. Maybe you started a new supplement.
- Know your inactive ingredients - if you’re allergic to lactose, gluten, or certain dyes, ask your pharmacist what’s in your generic. Not all labels list every filler, but they can look it up.
The Bigger Picture: Why This Matters
Generics make healthcare affordable. 90% of all prescriptions in the U.S. are filled with generics. That’s $254 billion saved every year. Without them, millions would skip doses or go without meds. The FDA’s 2023 AI pilot program analyzed 12.7 million prescriptions. It found no significant difference in interaction rates between brand and generic versions - except where patients switched manufacturers too often or misunderstood their meds. The real issue isn’t science. It’s communication. Patients aren’t being told that generics are identical in effect. They’re being told they’re cheaper - and that’s enough to make them doubt. The American Pharmacists Association now trains pharmacists to spend 3.5 hours on patient education about generics. That’s not about chemistry. It’s about trust.Bottom Line: Generics Are Safe. But Stay Aware.
A generic drug doesn’t interact differently because it’s generic. The active ingredient is the same. The risks are the same. The warnings are the same. But your body is sensitive. Your environment changes. Your pharmacy switches suppliers. And your mind can play tricks. If you’re on a critical medication, stick with the same generic when you can. Ask questions. Track changes. Don’t assume the worst. And if something feels off - talk to your doctor or pharmacist. Don’t blame the pill. Look at the whole picture. Because the truth is simple: your health doesn’t care if the pill has a brand name or not. It only cares if the right medicine is in your system - and that’s exactly what generics deliver.Do generic drugs have the same drug interactions as brand-name drugs?
Yes. Generic drugs contain the exact same active ingredient as their brand-name counterparts and are required by the FDA to have identical interaction profiles. Any differences in side effects are almost always due to inactive ingredients, patient psychology, or changes in diet or other medications - not the generic status itself.
Can inactive ingredients in generics cause side effects?
Yes, but rarely. Inactive ingredients like fillers, dyes, or preservatives can trigger allergic reactions or digestive issues in sensitive individuals. For example, a generic levothyroxine containing lactose caused stomach distress in a patient allergic to dairy - while the brand version used a different filler. These are not drug interactions, but individual sensitivities.
Are generics less effective for narrow therapeutic index drugs like warfarin or levothyroxine?
No. Generics for these drugs must meet stricter FDA bioequivalence standards (90%-112% vs. the usual 80%-125%). However, switching between different generic manufacturers can introduce variability in inactive ingredients, which may affect absorption in sensitive patients. For this reason, doctors and hospitals often recommend sticking with one generic manufacturer for these drugs.
Why do some people feel worse after switching to a generic?
The most common reasons are the nocebo effect (expecting side effects because you believe generics are inferior), switching between different generic manufacturers with different fillers, or coincidental changes in diet, sleep, or other medications. Studies show that over 90% of reported differences are not due to pharmacological changes in the drug itself.
Should I avoid generics because of interaction risks?
No. Generics are safe, effective, and responsible for 90% of prescriptions in the U.S. Avoiding them due to fear of interactions costs billions annually and can lead to untreated conditions. If you’re concerned, talk to your pharmacist about consistency in manufacturer, check for allergens in inactive ingredients, and track your symptoms - but don’t assume the generic is the problem.





ian septian
December 11, 2025 AT 10:04Generics saved my life. I was on $600/month brand-name meds until I switched. Same results, $15 a month. Stop fearing what works.
George Taylor
December 12, 2025 AT 17:52Oh, so now it's 'just psychology'? You know, I've been on the same generic for 3 years... then one day, my hands started shaking, I couldn't sleep, and I felt like I was being slowly poisoned. My doctor said 'it's the nocebo effect.' I said, 'then why did it stop when I switched back to the brand?' ...and now I'm paying $400 a month to not feel like I'm dying. So yeah, maybe your science is right... but my body isn't wrong.
Evelyn Pastrana
December 14, 2025 AT 02:55Wow, so the system is designed to save us money... but our minds are the enemy? 😏 Meanwhile, my pharmacist just handed me a blue pill instead of the white one, and I'm supposed to just shrug and say 'it's fine'? Nah. I'll take the extra $10 to not feel like I'm hallucinating.
Chris Marel
December 14, 2025 AT 21:23I come from a country where generics are the only option, and I’ve seen people thrive on them. But I also know someone who had a bad reaction to a filler-lactose, actually. It’s not about distrust. It’s about awareness. If your body reacts, listen to it. Not because generics are bad-but because we’re all different.
Nikhil Pattni
December 16, 2025 AT 05:16Listen, I'm a pharmacologist from India and I've reviewed over 200 bioequivalence studies. The FDA’s 80-125% range? Totally fine for 98% of drugs. But for NTI drugs like warfarin, even 5% variation can be dangerous. And guess what? In India, they don't even test for that! We have generics that are 75% bioavailable and people die because they think 'it's the same'. So don't tell me it's all in your head. In some countries, it's literally not the same. And even in the U.S., when pharmacies switch manufacturers without telling you? That's not transparency-that's negligence. I've seen patients on warfarin with INR levels jump from 2.1 to 5.8 because their pharmacy switched from Actavis to Mylan and no one warned them. This isn't psychology. It's supply chain chaos.
Arun Kumar Raut
December 16, 2025 AT 11:50My mom’s on levothyroxine. She switched generics and felt tired for two weeks. We called the pharmacy-they switched brands. We asked to stick with the same one. Now she’s fine. No drama. Just ask. It’s that simple.
Carina M
December 17, 2025 AT 12:25It is profoundly irresponsible to suggest that patient-reported adverse effects are merely the product of psychological fallacy. One does not simply dismiss the phenomenological experience of the individual in favor of statistical aggregates. The body does not lie. The data may obscure, but the lived reality persists. To reduce human suffering to a cognitive bias is the very epitome of medical paternalism.
William Umstattd
December 19, 2025 AT 01:46So let me get this straight: the FDA says generics are identical, but patients still feel worse-so it’s their fault? That’s like saying a car’s engine is fine, but if you feel the steering shake, it’s just your imagination. No. If the damn wheel is wobbling, fix the damn wheel. Stop blaming the driver. And stop pretending the system is flawless just because the numbers look good on paper.
Elliot Barrett
December 19, 2025 AT 19:35Why are we even having this conversation? If you’re on warfarin and your pharmacy switches your generic without telling you? That’s malpractice. Not ‘nocebo.’ Not ‘perception.’ That’s a systemic failure. Someone needs to get sued.
Courtney Black
December 20, 2025 AT 01:06It’s not about the pill. It’s about control. We live in a world where everything is commodified-even our biology. We’re told to trust the system, but the system doesn’t trust us. It doesn’t tell us when the pill changes. It doesn’t warn us about fillers. It just says ‘it’s the same.’ But we’re not machines. We’re humans who remember how we felt last month, who notice the color of the capsule, who feel the silence when the pharmacist won’t look us in the eye. So maybe the real interaction isn’t between drugs… it’s between power and vulnerability.